

Continuously create new value for health

Scientific and Technological Achievements Reserve/Project Establishment/Research and Development/Production System Establishment/Product Testing/Animal Testing/Clinical Trials/Innovation Application/Product Registration/Contract Manufacturing

Passed Center for Medical Device Evaluation of NMPA Master File Registration Record(M2022063-000)
We are committed to becoming a leader of Marketing Authorization solutions for the medical industry and continues to create new value for health.
Beijing Dikang Pharmaceutical Investment Management Co., Ltd. provides global customers with one-stop Tissue Engineering and Regenerative Medicine Contract Development and Manufacturing solutions.The company is committed to becoming a leader of marketing authorization solutions for the medical industry to continuously create new value for health.At present, we have reserved more than 30 technical projects, obtained five national invention patent certificates, and three medical device registration certificates, three China's first scientific research achievements, and completed one medical device raw material master file registration.And we have won many honours such as "National High-Tech Enterprise", "Beijing Innovative Small and Medium-sized Enterprise" and "Beijing ‘Specialized and Sophisticated’ Small and Medium-sized Enterprise."

Scientific and Technological Achievements Reserves
National Invention Patents
Medical Device Registration Certificates
Technology Transformation
We have mainly undertaken in the research and development, clinical trials and product registration of ‘low immunogenicity, high stability collagen and silk fibroin engineered products’.

 Contract Development and Manufacturing
Contract Development and Manufacturing
Dikang Pharma is a one-stop CDMO service supplier for the conversion of the high-quality medical device scientific and technological achievements, providing a full range of solutions in medical device industry, from research and development, product testing, animal testing, clinical trials, product registration to contract manufacturing, to accelerate the efficiency of commercialisation of medical achievements.

 dECM Project Transfer and Authorisation
dECM Project Transfer and Authorisation
The Tissue Engineering and Regenerative Medicine Biomaterials Laboratory has undertaken the special project of "National 13th Five Year Key R&D Plan for Biomaterials Research and Development and Organ Repair Replacement", and has achieved the transformation of scientific and technological achievements in "Engineering preparation technology and product development of low immunogenicity collagen and silk fibroin".

 Raw Material Supply
Raw Material SupplyDecellularized extracellular matrix (dECM) is a natural material purified by removing cells, antigens and other components from animal or human tissues using different decellularisation techniques such as physical, chemical and biological.And it has the main components of extracellular matrix(ECM), possessing good cytocompatibility, biodegradability and tissue regeneration potential.
We are committed to becoming a leader of Marketing Authorization solutions for the medical industry and continues to create new value for health.

Recently, Guangdong Marubi Biotechnology Co., Ltd. had reached an agreement with Healtech Beijing CDMO Center. Marubi will directly acquire shares in Shengzhi Runhe, thereby expanding into the new field of extracellular matrix (ECM).

On 18th November, the new product launch of Hengan Fulin Lidian® Collagen Patch Dressing was successfully closed at the headquarters of Jingdong Health in Beijing.Leaders and experts from various parties witnessed this launch. This project is the first Class III medical device incubated by Healtech CDMO Center based on the whole process of ‘Marketing Authorization solution’ and formally entered into the commercialisation stage, which has an important milestone significance.It is the world's first new product that applies new medical material Extracellular Matrix (ECM) to the field of medical aesthetics in a large-scale and industrialised manner, and it sets off the clinical application of extracellular matrix .
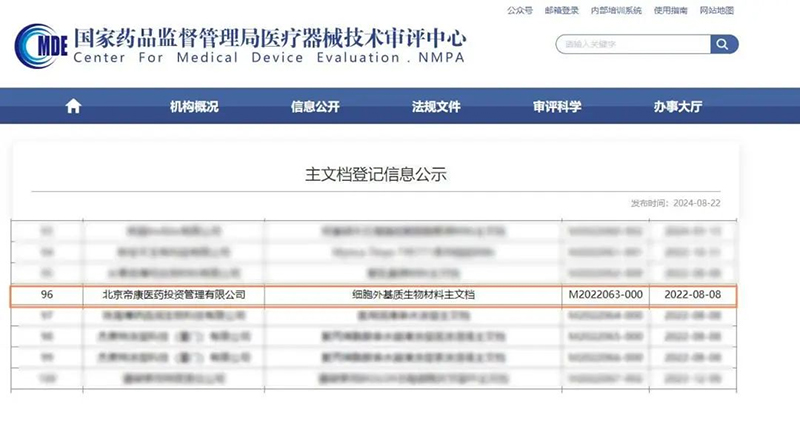
Dikang Pharma's decellularized extracellular matrix (d-ECM) had obtained invention patent authorization from the China National Intellectual Property Administration and master file registration from the National Medical Products Administration.
Healtech Beijing CDMO Center has been recognized as the Beijing “Specialized and Sophisticated” Small and Medium-sized Enterprise - It is another important qualification after National High-tech Enterprise, Zhongguancun High-tech Enterprise and Beijing Innovative Small and Medium-sized Enterprise.
2024/08/13On April 10th, Beijing Municipal Bureau of Economy and Information Technology issued the ‘Notice on Disclosure of the List of Innovative Small and Medium-sized Enterprises in Beijing in February 2024’. Healtech Beijing CDMO Center was listed among them, and was successfully awarded the title of ‘Beijing Innovative Small and Medium-sized Enterprises ’.
2024/05/30On 28th November 2023, Beijing Soyoung Technology Co., Ltd and Beijing Healtech Pharmaceutical Technology Co., Ltd held a signing ceremony for strategic cooperation in Beijing, to open a new chapter of deep-level cooperation between the two companies. Xing Jin, Ch
2023/12/01Copyright ©2020 www.dkbiotech.com.cn , All Rights Reserved
BeijingICPNo.20001702号-1![]() Jiangsu Computer Information Network and internet unit registration 11011502006138号 Qualification Certificate for Medicine Trading Services on the Internet:(Beijing)-Non-Operating-2020-0118
Jiangsu Computer Information Network and internet unit registration 11011502006138号 Qualification Certificate for Medicine Trading Services on the Internet:(Beijing)-Non-Operating-2020-0118